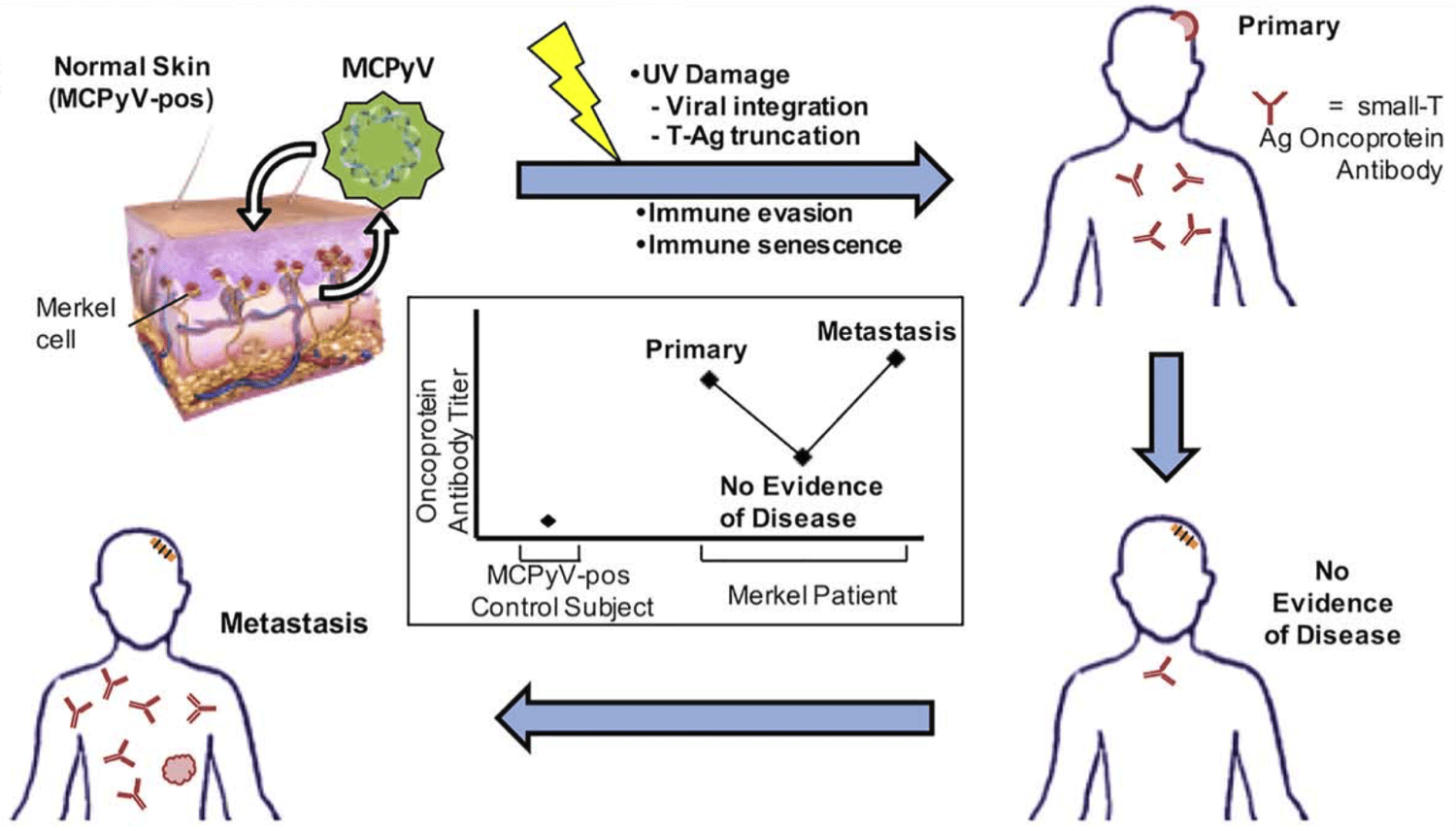
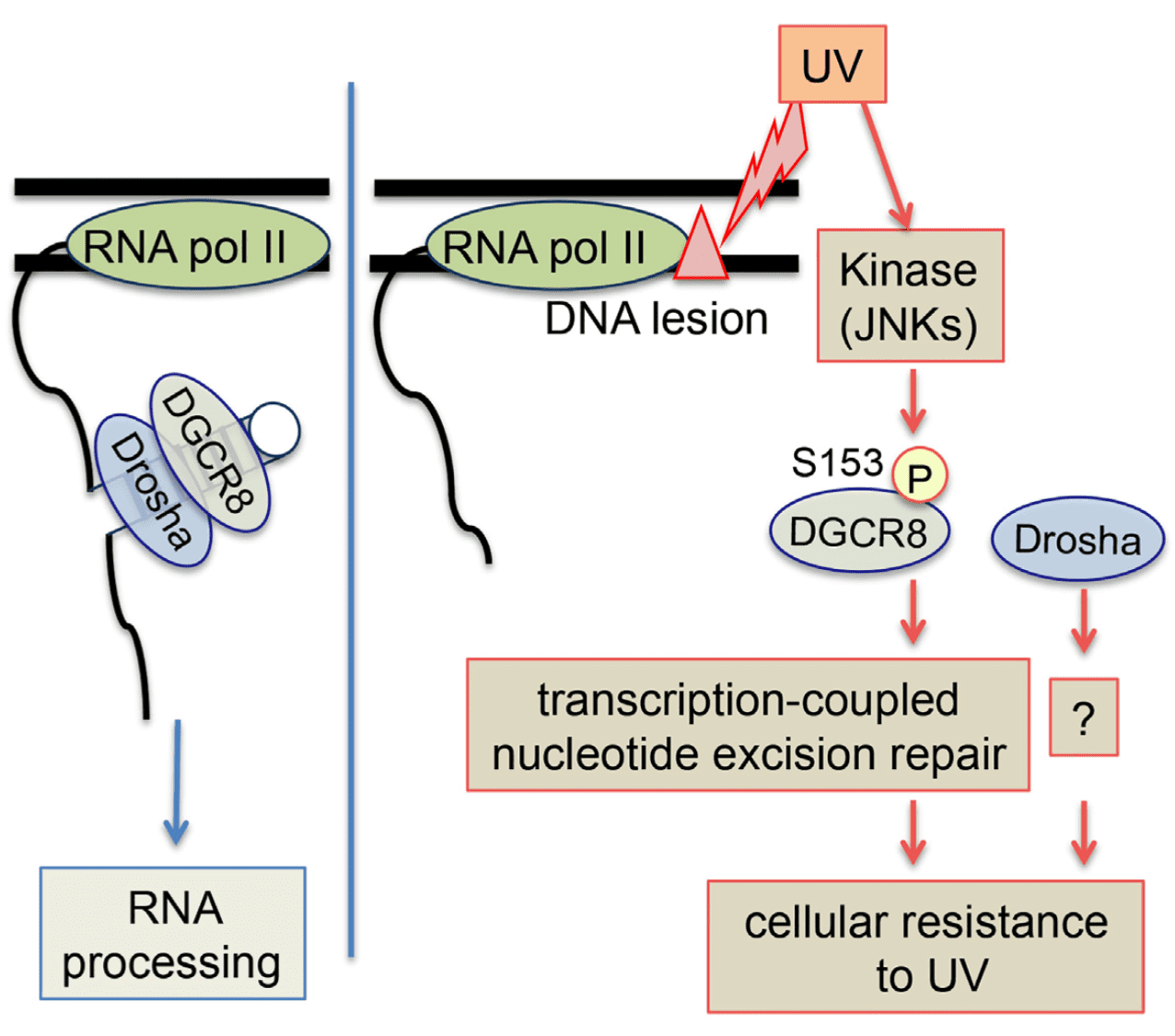
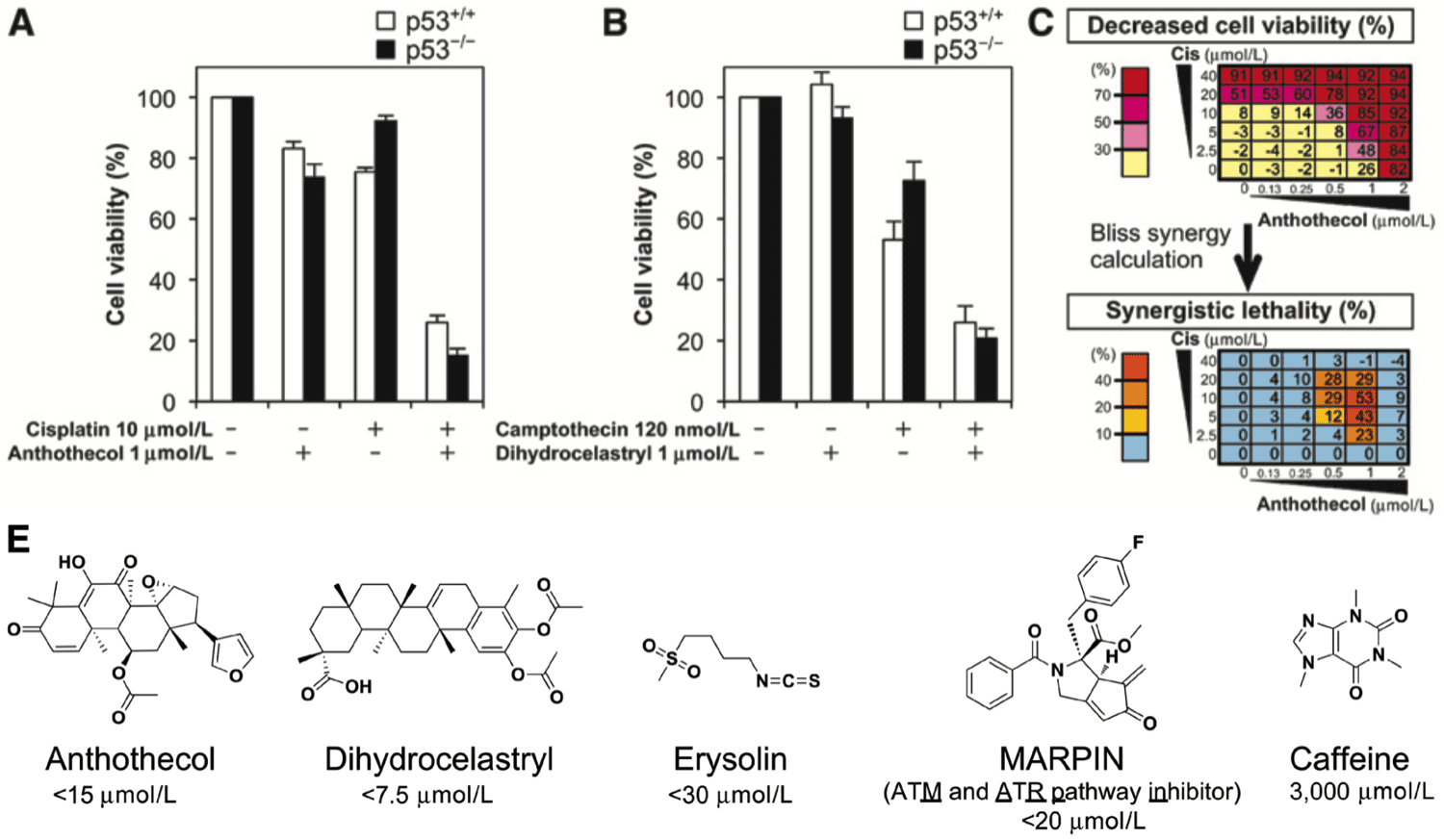
Highlights Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy with a recurrence rate of…
Abstract
Antibodies against APP, a precursor of Aβ deposited in Alzheimer’s disease brain, have been shown to cause neuronal death. Therefore, it is important to determine whether Aβ mediates antibody-induced neurotoxicity. When primary neurons were treated with anti-APP antibodies, Aβ40 and Aβ42 in the cultured media were undetectable by an assay capable of detecting 100 nM Aβ peptides. However, exogenously treated Aβ1-42 or Aβ1-43 required >3 µM to exert neurotoxicity, and 25 µM Aβ1-40 was not neurotoxic. Glutathione-ethyl-ester inhibited neuronal death by anti-APP antibody, but not death by Aβ1-42, whereas serum attenuated toxicity by Aβ1-42, but not by anti-APP antibody. Using immortalized neuronal cells, we specified the domain responsible for toxicity to be cytoplasmic His657-Lys676, but not the Aβ1-42 region, of APP. This indicates that neuronal cell death by anti-APP antibody is not mediated by secreted Aβ.