Highlights UV radiation induces phosphorylation on S153 of DGCR8. Phosphorylation on S153 of DGCR8 mediates…
Highlights
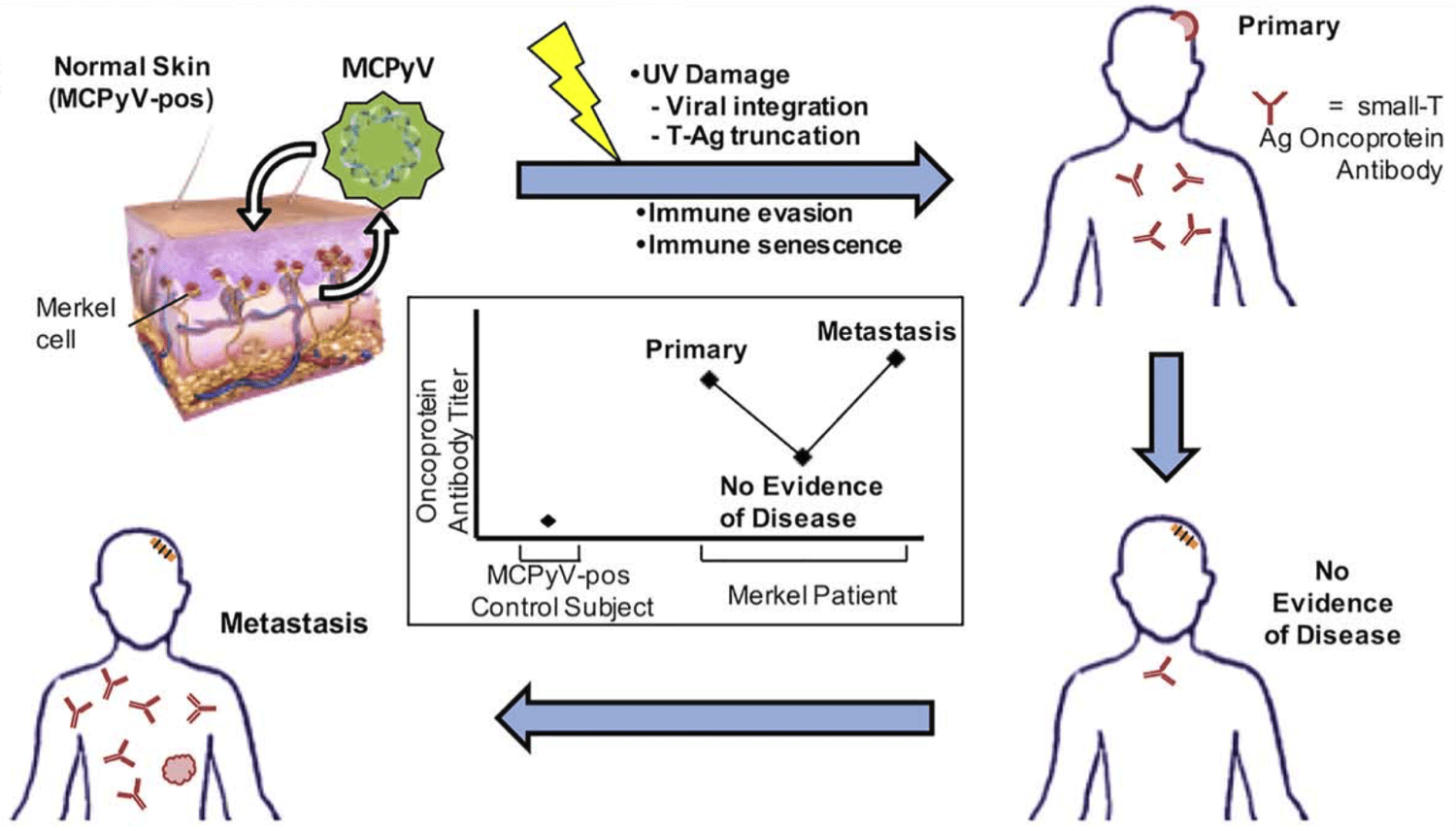
- Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy with a recurrence rate of >40%, and a causative polyomavirus [Merkel cell polyomavirus (MCPyV)] was identified in 80% of MCCs.
- We tested 2 clinical roles for MCPyV-oncoprotein antibody quantification: for initial MCC prognostication and as a marker for disease recurrence after definitive therapy.
- Greater than 50% of patients with MCC make MCPyV-oncoprotein antibodies, and those who do not are at higher risk for recurrence and may benefit from closer follow-up with imaging.
- MCPyV-oncoprotein antibodies have clinical utility for the early detection of occult recurrent or distant metastatic disease.
Abstract
BACKGROUND
Merkel cell carcinoma (MCC) is an aggressive skin cancer with a recurrence rate of >40%. Of the 2000 MCC cases per year in the United States, most are caused by the Merkel cell polyomavirus (MCPyV). Antibodies to MCPyV oncoprotein (T-antigens) have been correlated with MCC tumor burden. The present study assesses the clinical utility of MCPyV-oncoprotein antibody titers for MCC prognostication and surveillance.
METHODS
MCPyV-oncoprotein antibody detection was optimized in a clinical laboratory. A cohort of 219 patients with newly diagnosed MCC were followed prospectively (median follow-up, 1.9 years). Among the seropositive patients, antibody titer and disease status were serially tracked.
RESULTS
Antibodies to MCPyV oncoproteins were rare among healthy individuals (1%) but were present in most patients with MCC (114 of 219 patients [52%]; P < .01). Seropositivity at diagnosis independently predicted decreased recurrence risk (hazard ratio, 0.58; P = .04) in multivariate analyses adjusted for age, sex, stage, and immunosuppression. After initial treatment, seropositive patients whose disease did not recur had rapidly falling titers that became negative by a median of 8.4 months. Among seropositive patients who underwent serial evaluation (71 patients; 282 time points), an increasing oncoprotein titer had a positive predictive value of 66% for clinically evident recurrence, whereas a decreasing titer had a negative predictive value of 97%.
CONCLUSIONS
Determination of oncoprotein antibody titer assists in the clinical management of patients with newly diagnosed MCC by stratifying them into a higher risk seronegative cohort, in which radiologic imaging may play a more prominent role, and into a lower risk seropositive cohort, in which disease status can be tracked in part by oncoprotein antibody titer.