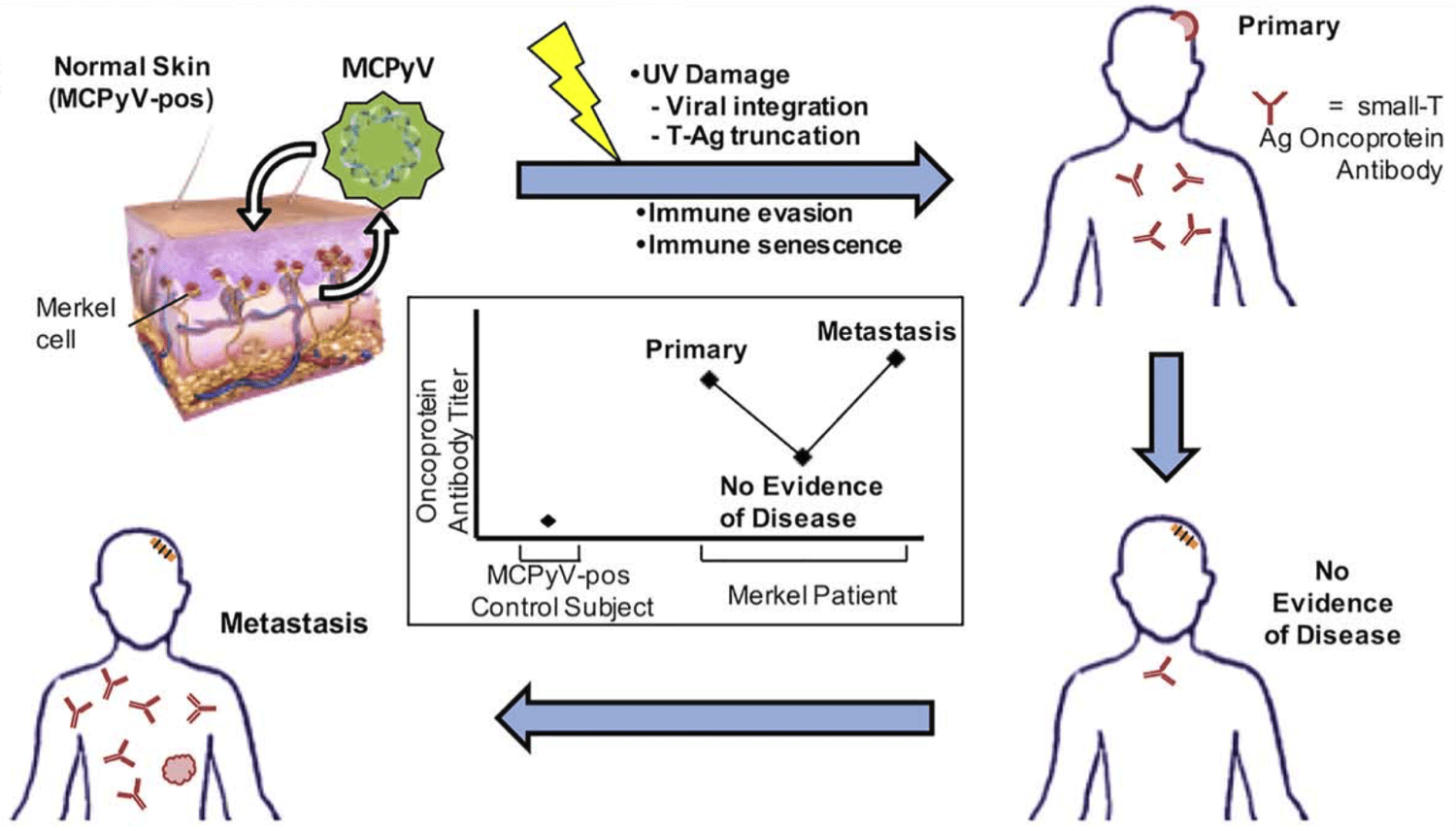
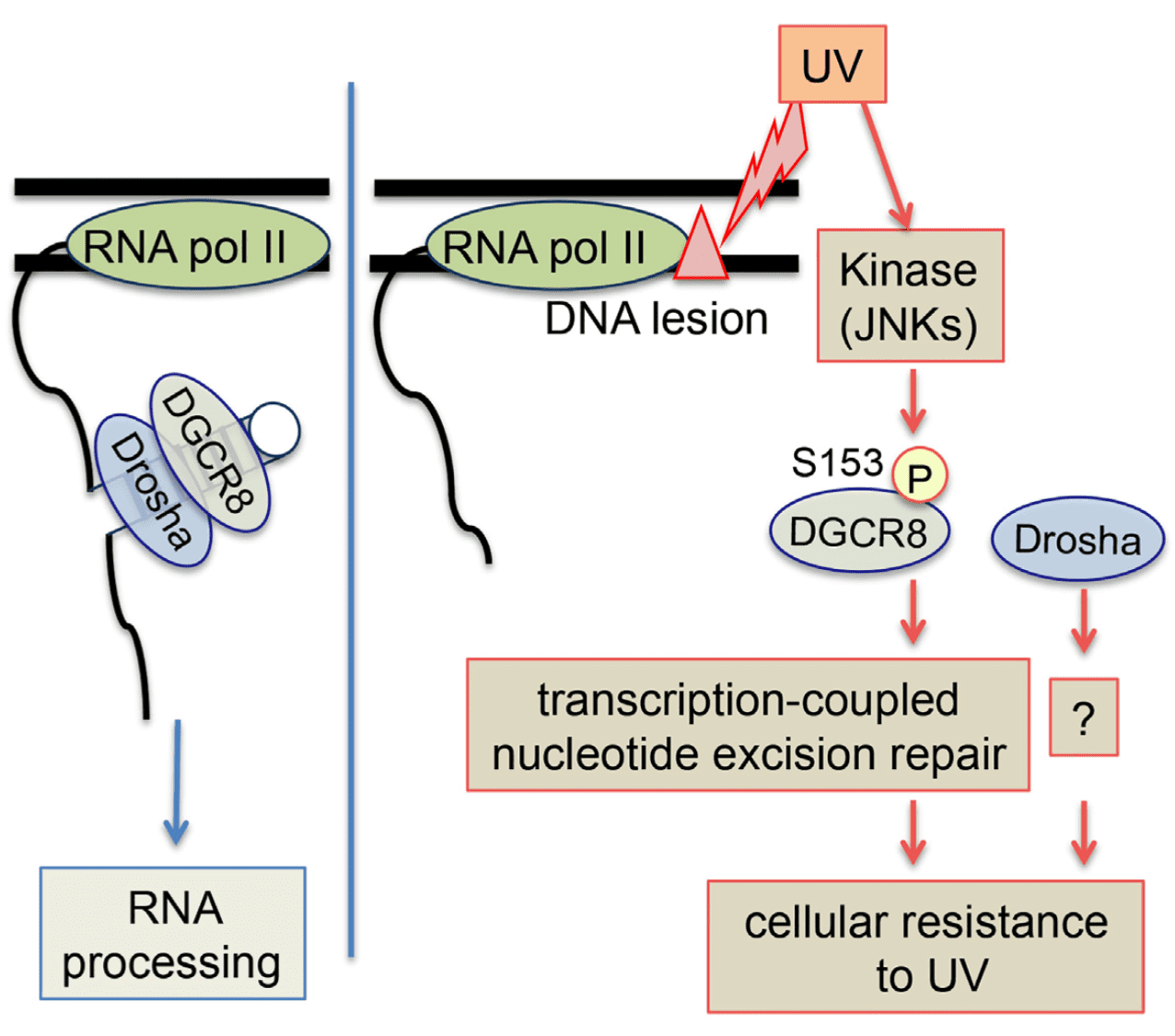
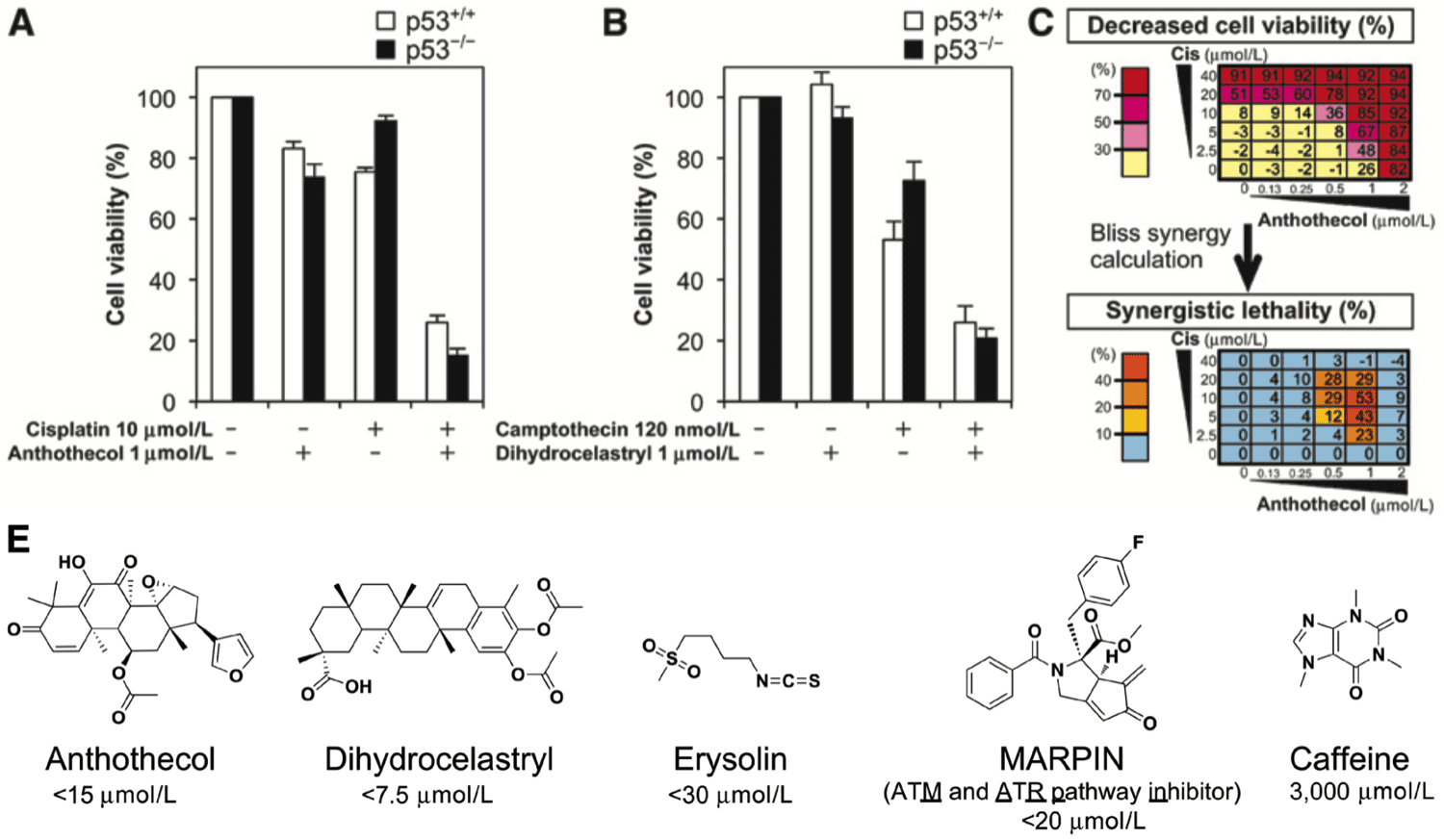
Highlights Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy with a recurrence rate of…
Abstract
Using a yeast two-hybrid method, we searched for amyloid precursor protein (APP)-interacting molecules by screening mouse and human brain libraries. In addition to known interacting proteins containing a phosphotyrosine-interaction-domain (PID)-Fe65, Fe65L, Fe65L2, X11, and mDab1, we identified, as a novel APP-interacting molecule, a PID-containing isoform of mouse JNK-interacting protein-1 (JIP-1b) and its human homolog IB1, the established scaffold proteins for JNK. The APP amino acids Tyr682, Asn684, and Tyr687 in the G681YENPTY687 region were all essential for APP/JIP-1b interaction, but neither Tyr653 nor Thr668 was necessary. APP-interacting ability was specific for this additional isoform containing PID and was shared by both human and mouse homologs. JIP-1b expressed by mammalian cells was efficiently precipitated by the cytoplasmic domain of APP in the extreme Gly681-Asn695 domain-dependent manner. Reciprocally, both full-length wild-type and familial Alzheimer’s disease mutant APPs were precipitated by PID-containing JIP constructs. Antibodies raised against the N and C termini of JIP-1b coprecipitated JIP-1b and wild-type or mutant APP in non-neuronal and neuronal cells. Moreover, human JNK1β1 formed a complex with APP in a JIP-1b-dependent manner. Confocal microscopic examination demonstrated that APP and JIP-1b share similar subcellular localization in transfected cells. These data indicate that JIP-1b/IB1 scaffolds APP with JNK, providing a novel insight into the role of the JNK scaffold protein as an interface of APP with intracellular functional molecules.