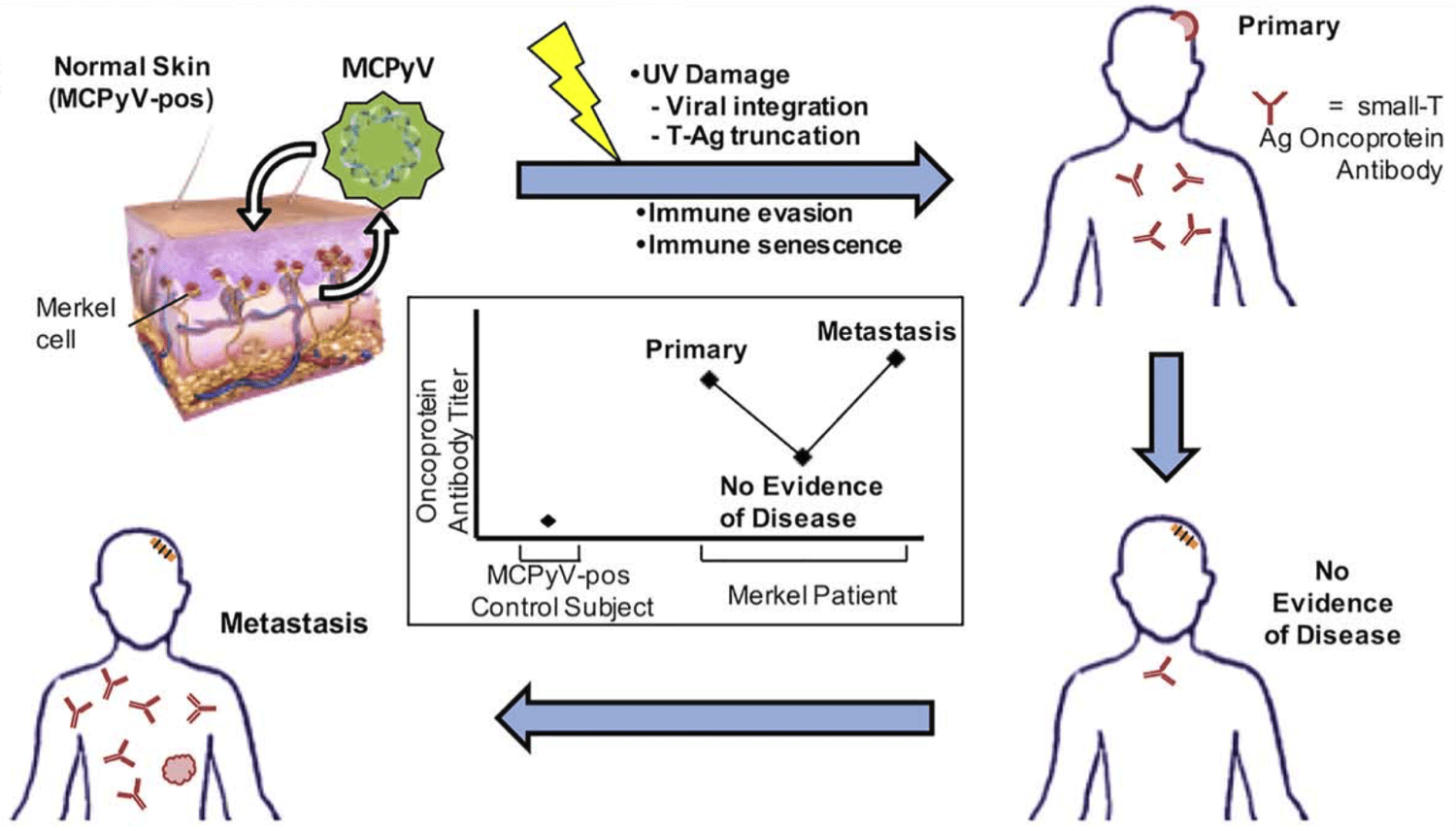
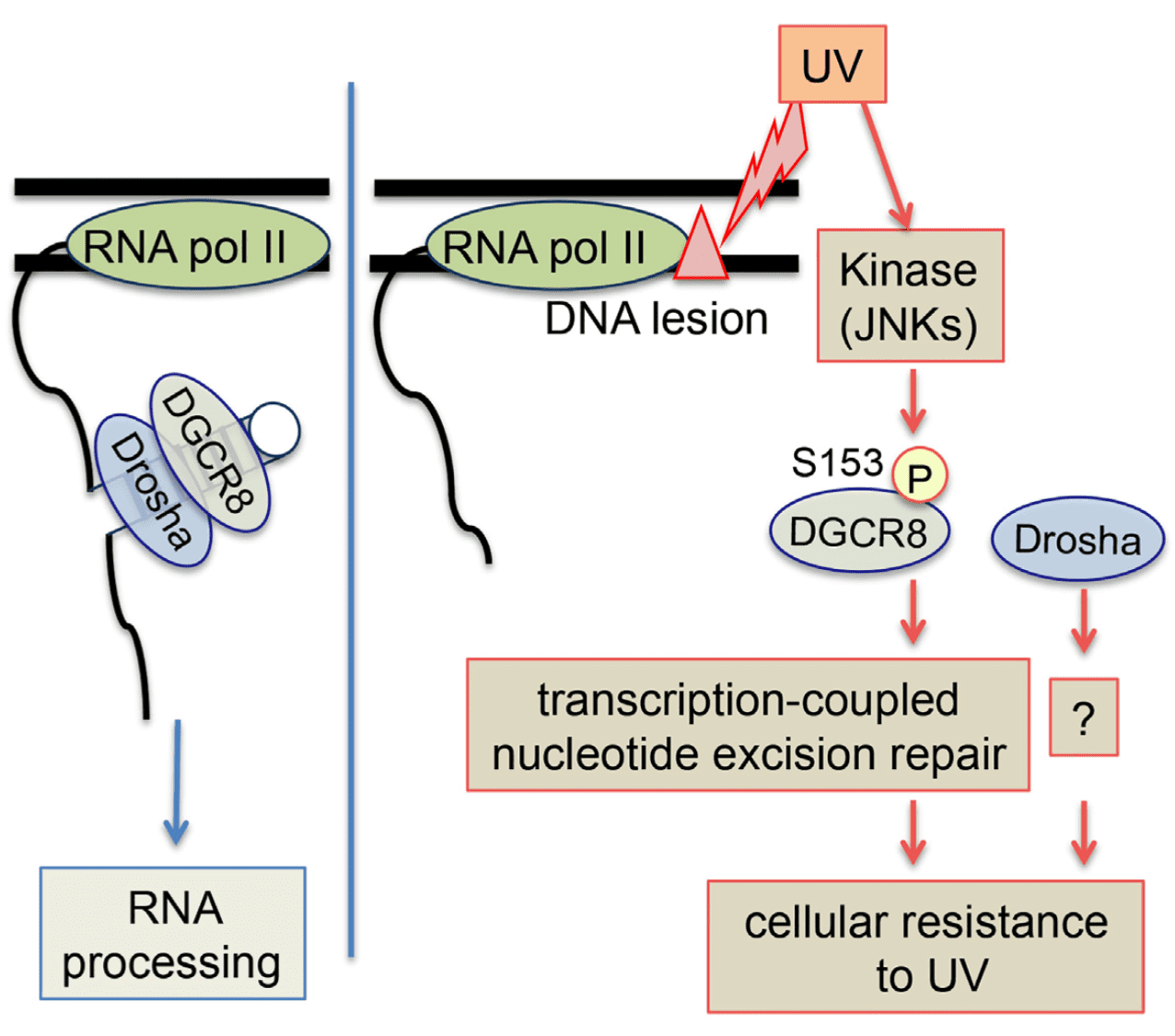
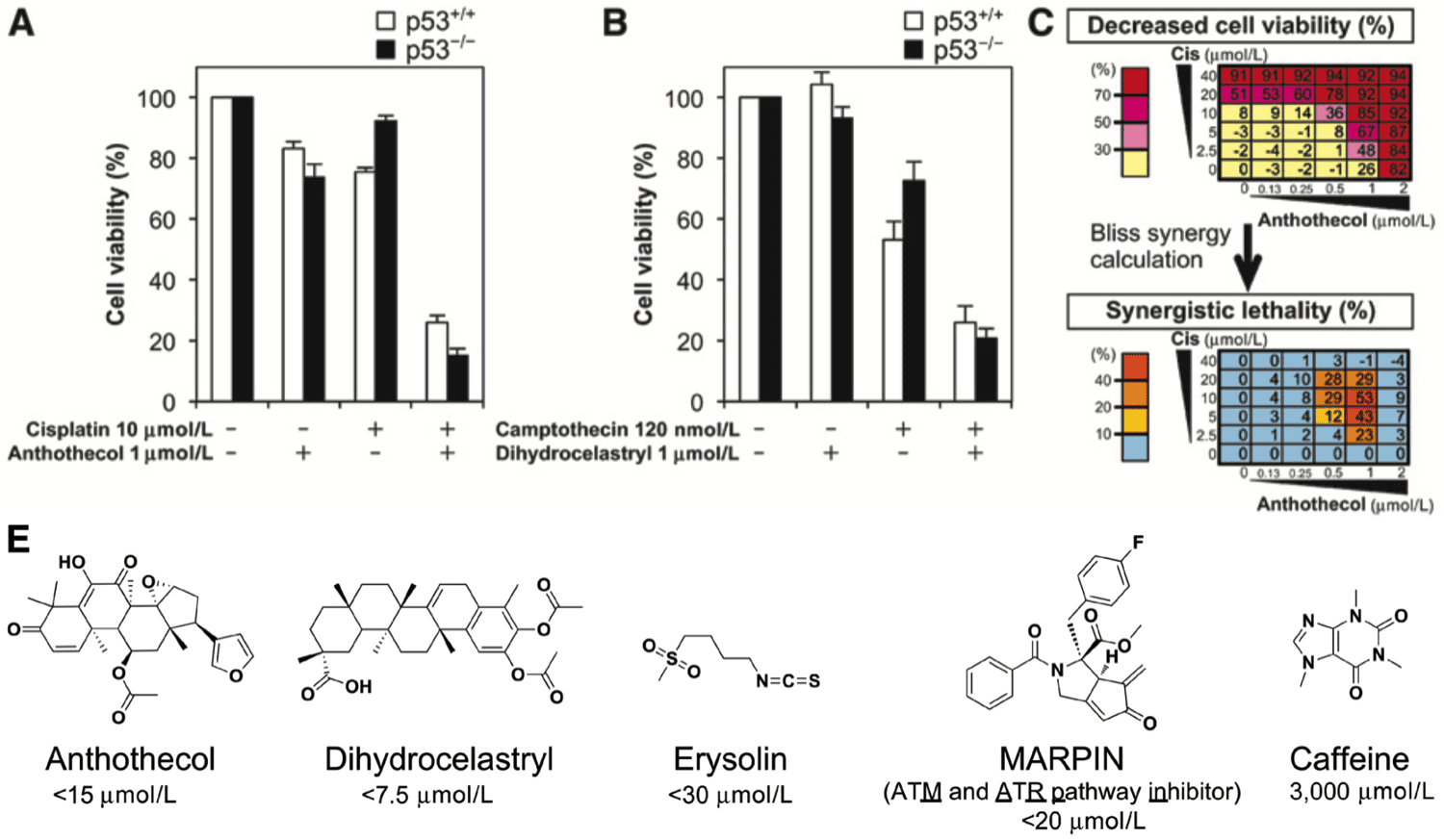
Highlights Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy with a recurrence rate of…
Abstract
Alzheimer’s disease is characterized pathologically by senile plaques in the brain. The major component of senile plaques is amyloid-β (Aβ), which is cleaved from Alzheimer’s Aβ protein precursor (AβPP). Recently, information regarding the cytoplasmic tail of AβPP has started to emerge, opening up various insights into the physiological roles of AβPP and its pathological role in Alzheimer’s disease. The cytoplasmic domain of AβPP shares the evolutionarily conserved GYENPTY motif, which binds to a number of adaptor proteins containing the phosphotyrosine interaction domain (PID). Among the PID-containing proteins, this article focuses on four groups of adaptor proteins of AβPP: Fe65, X11, mDab1, and c-Jun N-terminal kinase-interacting protein 1b/islet-brain 1.