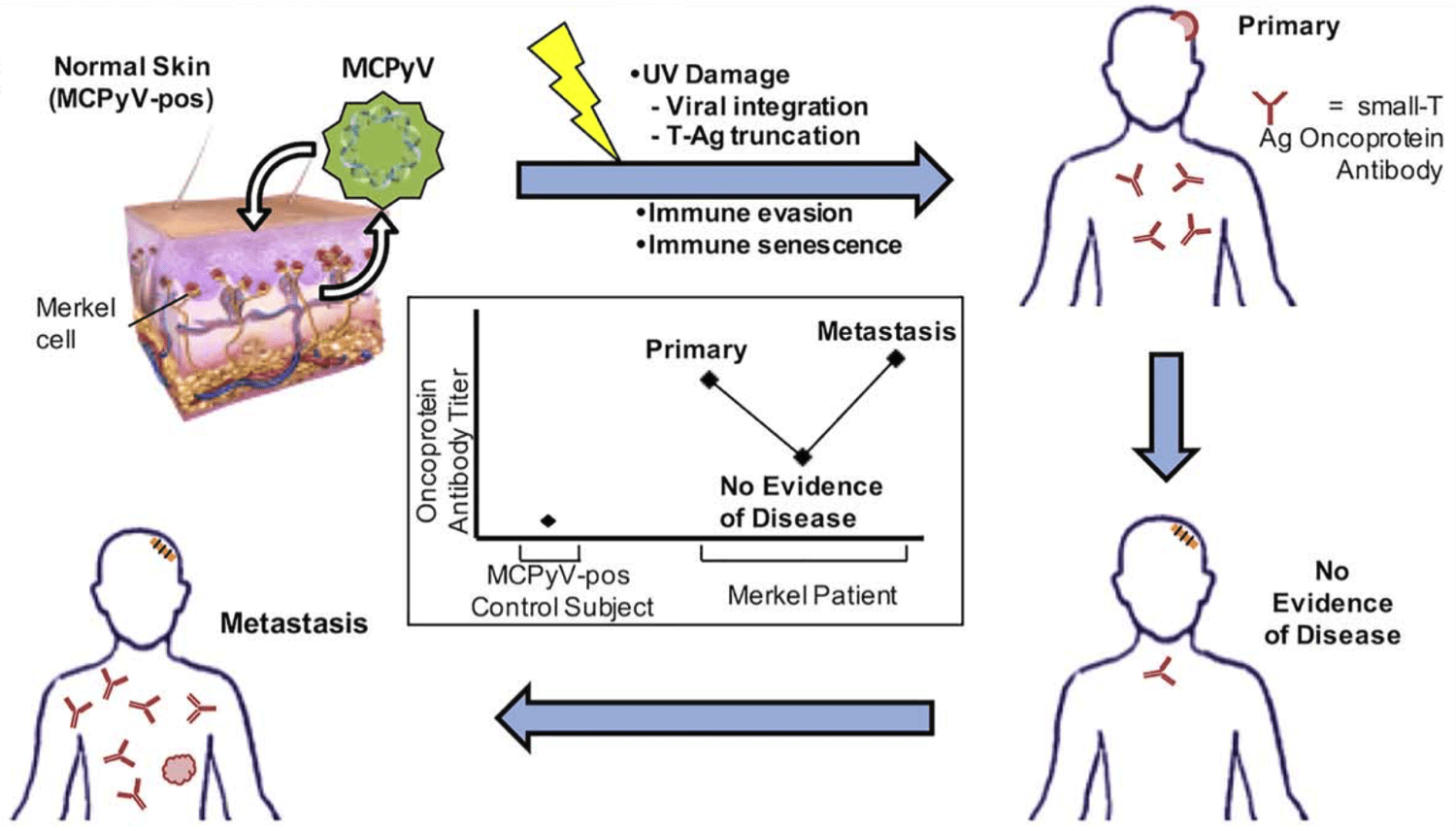
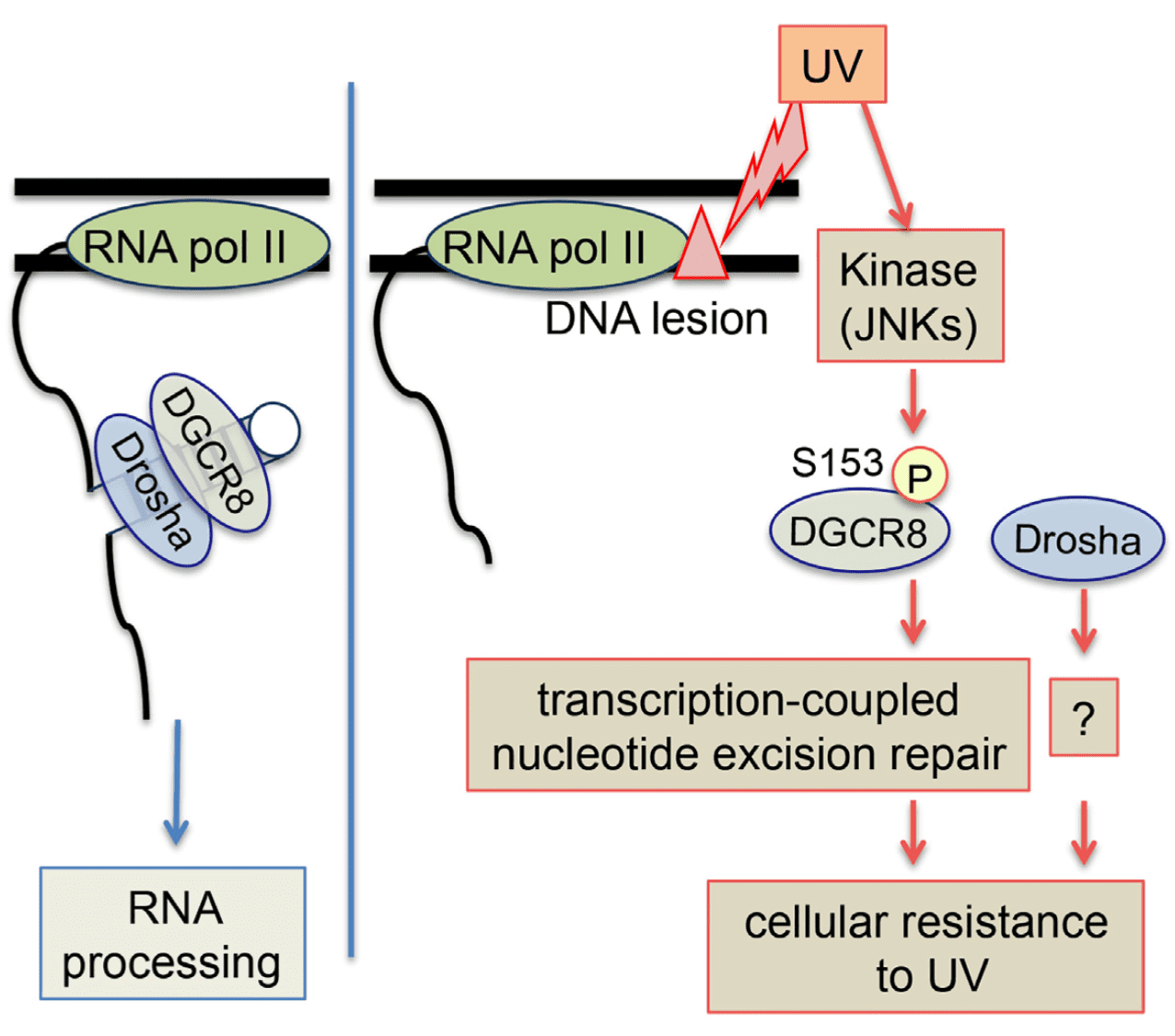
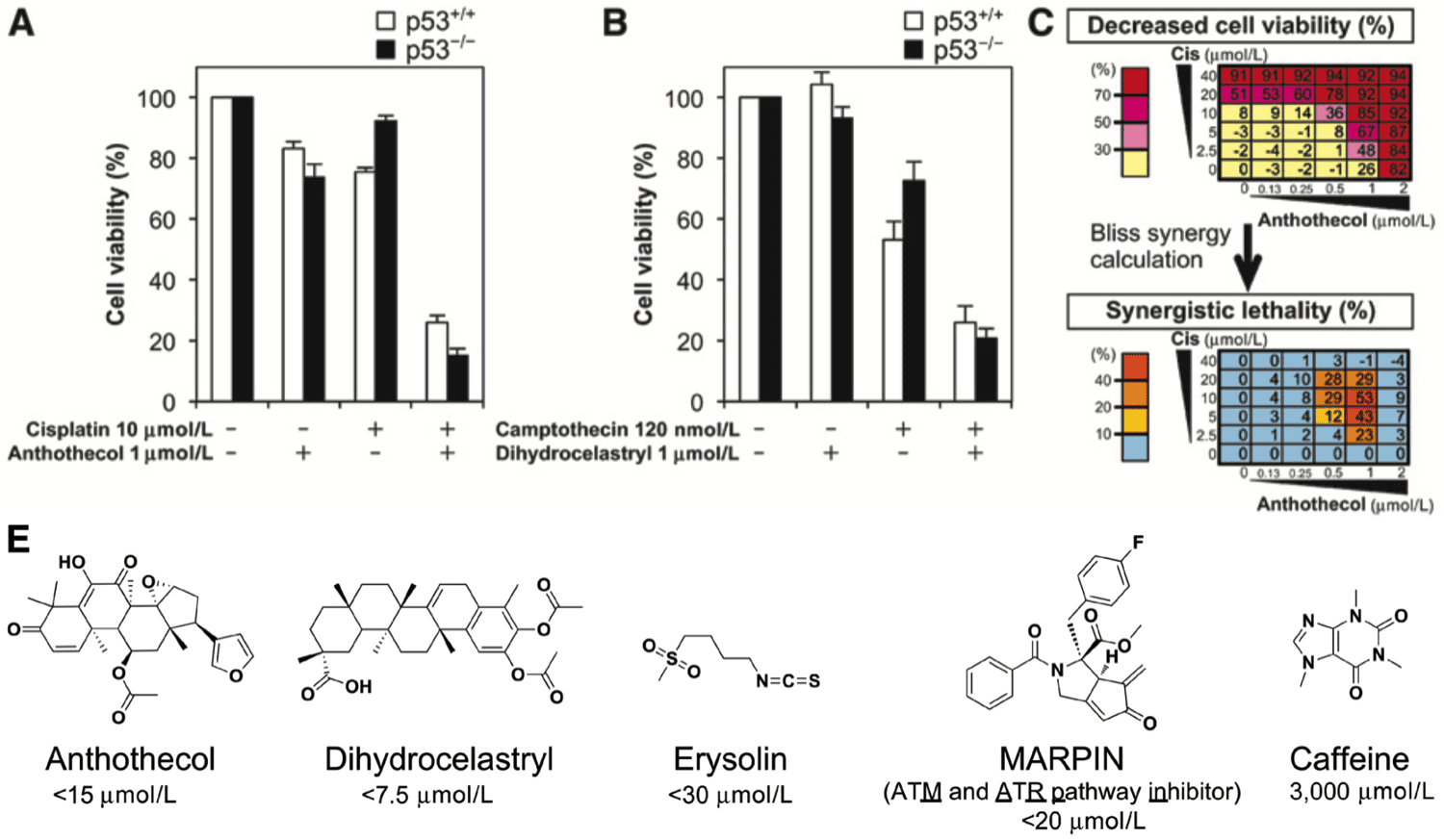
Highlights Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy with a recurrence rate of…
Abstract
Humanin (HN) is a 24-amino acid peptide that protects neuronal cells from death caused by Alzheimer’s disease (AD)-related genes and amyloid-β (Aβ). Multiple studies have revealed its biochemical and neuroprotective characteristics in vitro; however, little has been known regarding whether HN is effective in vivo in AD model systems. We examined the effect of S14G-HN, a 1,000-fold more potent derivative of HN in vitro, on amnesia induced by Aβ 25-35 in mice. The Y-maze test revealed that at least 50 pmol of S14G-HN by intracerebroventricular injection prevented Aβ-induced impairment of short-term/spatial working memory; however, 5 nmol of S14A-HN, a neuroprotection-defective mutant in vitro, did not prevent Aβ -induced amnesia. These results are in agreement with the structure-function correlation shown previously in vitro. In the water-finding task, S14G-HN prevented prolongation of finding latency (the time to find water) observed in Aβ-amnesic mice, indicating that S14G-HN also blocked Aβ -induced impairment of latent learning. In accordance with these observations, immunohistochemical analysis showed that S14G-HN sustained the number of cholinergic neurons in the basal forebrain and the striata nearly to the normal level. Furthermore, genistein, a specific inhibitor of tyrosine kinases, blocked recovery from scopolamine-induced amnesia by S14G-HN, suggesting that certain tyrosine kinase(s) are involved in the inhibitory function of S14G-HN in vivo. Taking these findings together, we conclude that S14G-HN has rescue activity against memory impairment caused by AD-related insults in vivo by activating the same intracellular neuroprotective machinery as elucidated previously in vitro.